| Reactions |
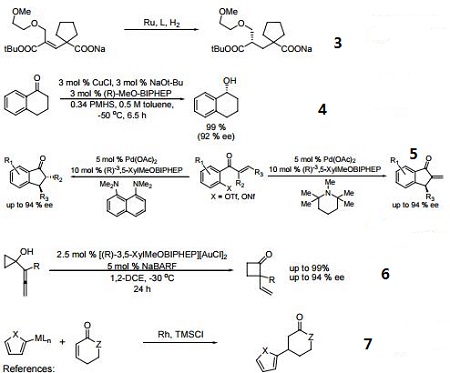
In many respects the catalytic profile of the MeOBIPHEP ligands is similar to that of other atropisomeric diphosphines such as binap and its many analogs. The nature of the PR2 group strongly influences the catalytic performance of the metal complexes. The rhodium and ruthenium MeO-BIPHEP catalysts are highly effective for the hydrogenation of various C=O, C=C and C=N bonds and several synthetically useful C-C coupling reactions.
- Ru and Ir catalyzed dynamic kinetic resolution for the synthesis of hydroxy, amino acid derivatives.
- Ru-catalyzed asymmetric hydrogenation of ketones and alkenes.
- Enantioselective copper-catalyzed asymmetric hydrosilylation of aryl ketones.
- Synthesis of chiral 3-substituted indanones via an enantioselective reductive-Heck reaction.
- Gold(I)-catalyzed enantioselective ring expansion of allenylcyclopropanols.
- Conjugate addition using 2-heteroaryl titanates and zinc reagents.
 |
|
|
| Description |
Platinum was discovered in Colombia, South America by Ulloa in 1735 and six years later in 1741 by Wood. The metal was isolated from native platinum by Delisle in 1775 and produced in malleable form by Chabaneau in 1786. Wollaston in 1803 developed a method of obtaining pure malleable platinum from crude platinum by extraction with aqua regia. The process led to the discovery of two other platinum group metals, palladium and rhodium, that were found in the aqua regia extract after platinum precipitated. Platinum derived its name from platina originating from the Spanish word plata for silver, because it was thought to be a trivial unwanted material associated with gold in gold mines of Central America.
Platinum occurs in nature as a bright-white cubic crystalline solid with metallic luster associated with other noble metals of its group. Platinum also occurs as the mineral sperrylite, PtAs2, found as tin-white brittle cubic crystals containing 52−57% platinum in certain nickel-bearing deposits. Some other minerals of platinum are cooperite PtS (Pt 80-86%); and braggite(Pt, Pd, Ni)S (Pt 58-60%). The abundance of platinum in the earth’s crust is estimated to be 0.005 mg/kg. |
|
|
| Chemical Properties |
Black Powder |
|
|
| Uses |
Platinum metal and its alloys have numerous applications. As a precious metal it is used extensively in jewelry. Other important applications include construction of laboratory crucibles and high temperature electric furnaces; in instruments as thermocouple elements; as wire; for electrical contacts; as electrodes; in dentistry; in cigarette lighters; and for coating missile and jet engine parts.
Platinum also is used extensively as a catalyst in hydrogenation, dehydrogenation, oxidation, isomerization, carbonylation, and hydrocracking. Also, it is used in organic synthesis and petroleum refining. Like palladium, platinum also exhibits remarkable ability to absorb hydrogen. An important application of platinum is in the catalytic oxidation of ammonia in Ostwald's process in the manufacture of nitric acid. Platinum is installed in the catalytic converters in automobile engines for pollution control. |
|
|
| Uses |
manufacture of apparatus for laboratory and industrial use, thermocouples, platinum resistance thermometers, acidproof containers, electrodes, etc. In dentistry; jewelry; electroplating. As oxidation catalyst in manufacture of acetic acid, nitric acid from ammonia, manufacture of sulfuric acid; control of automotive emissions. |
|
|
| General Description |
Silvery, whitish-gray, malleable, ductile metal. Mp: 1772°C; bp: 2187°C. Density: 21.45 g cm-3 at room conditions (very dense). Also shipped as a finely divided powder (Platinum black), as a sponge, and as particles deposited on a supporting material such as alumina. Has strong catalytic activity in these forms; finely divided Platinum can be dangerous to handle in the vicinity of other chemicals on this account. Used Platinum catalysts are particularly dangerous and can be explosive. |
|
|
| Reactivity Profile |
Massive Platinum (lump, ingot, etc.) is generally inert. Dissolves readily in aqua regia (mixture of concentrated hydrochloride and concentrated nitric acids). Reacts rapidly with molten alkali metal oxides and peroxides. Reacts with F2 and Cl2 at red heat. Absorbs large volumes of hydrogen when hot. Catalyzes the exothermic oxidation of ammonia by air. Finely divided Platinum is incompatible with aluminum, acetone, arsenic, ethane, hydrazine, hydrogen peroxide, lithium, phosphorus, selenium, tellurium and many fluorides. Explosion can occur upon contact with hydrogen peroxide. Platinum black, sponge and supported catalysts have strong catalytic activity; can be dangerous to handle in the vicinity of other chemicals on this account. Used Platinum catalysts are particularly dangerous and can cause explosions. Ethanol or methanol can ignite on contact with a Platinum-black catalyst. (Urben 1794). |
|
|